Books by Author Lovekush Kumar
Explore the books by author for excelling in your discipline.
Read NowAppreciation and Promotion
Explore for "promotable images/quotes" at your social media for a better society.
Read Now

Recent Articles
Four objects of mass 1 kg are placed at the vertices of a square of side 2 m, an axis passing perpendicular to the plane of the square through one of the vertices then calculate the moment of inertia about this axis ? JEE 2024
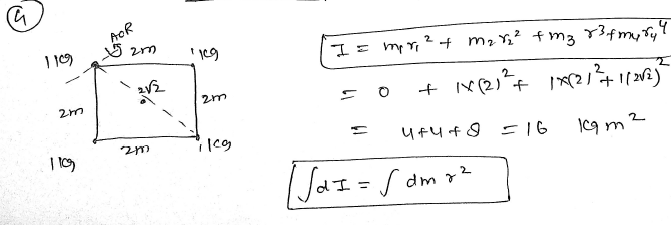
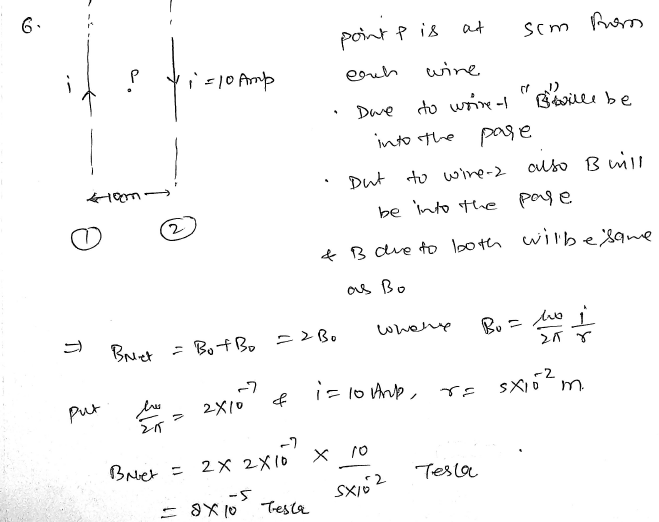
Two infinite long parallel wires kept at a distance of 10 𝑐𝑚, carrying equal currents in opposite directions of same magnitude 10 A. Find magnetic field in between the parallel wires. JEE 2024
If the particle undergoing SHM is having maximum velocity 10 cm/s and the amplitude of SHM is 4 cm. Find the distance of the particle from the mean position when the velocity of the particle is 5 cm/s? JEE 2024
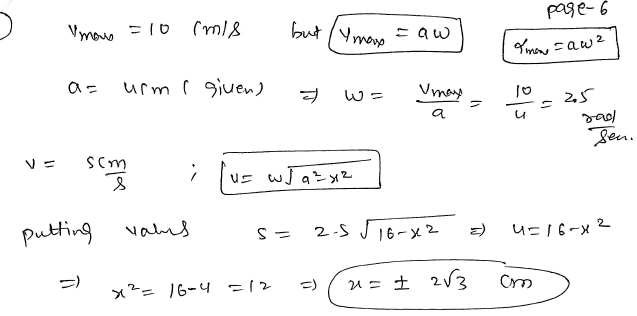
If the mass and specific heat of the body are 0.08 kg and 0.17 k cal/°𝐶. The temperature difference is 5°𝐶. Find the change in internal energy. JEE 2024
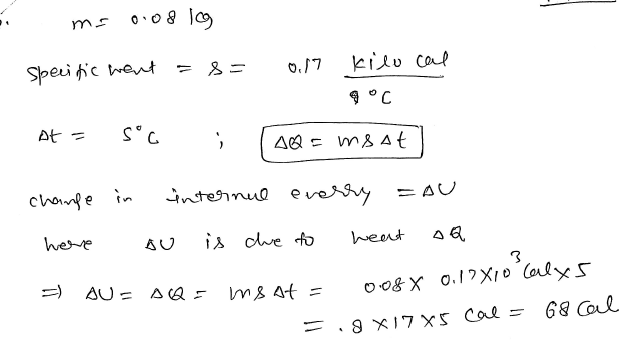
If displacement of the particle is S = 3𝑡^2 + 4𝑡 + 5 , then velocity a 𝑡=5 𝑠 will be? JEE 2024
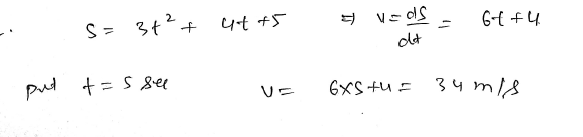
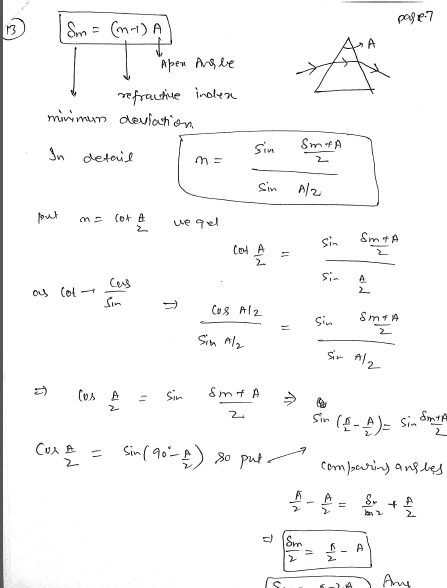
A prism has apex angle 𝐴 and refractive index 𝑛=cot(𝐴/2) . Find the minimum deviation? JEE 2024
minimum deviation = pi - 2A
Two masses 𝑚1 = 4 𝑔𝑚 and 𝑚2 = 25 𝑔𝑚 are having same kinetic energy, find the ratio of linear momentum ? JEE 2024

If the diameter of earth becomes half keeping mass to be constant, then the acceleration due to gravity at the surface of earth becomes ? JEE 2024
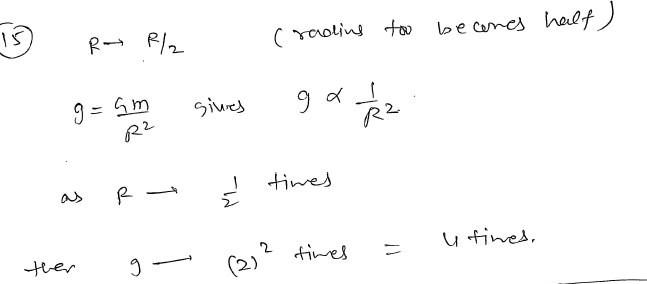
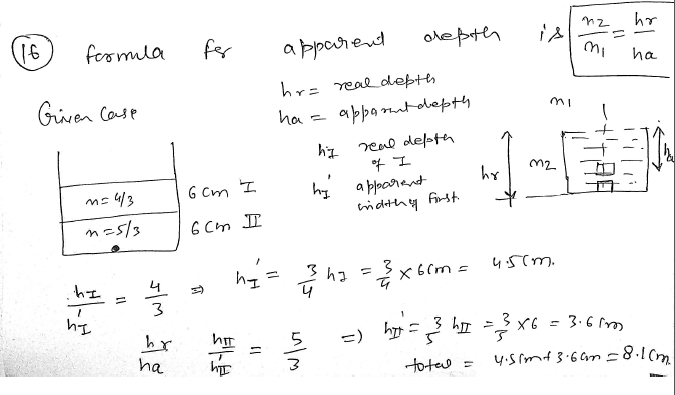
Find the apparent depth of a small object placed at the the bottom of two layers of 6 cm each one with refractive index of 4/3 and one with 5/3 ? JEE 2024
Apparent depth is real depth divided by refractive index of the liquid.